Researchers cast new light on muscle wasting diseases
Published On Mon 24 Mar 2014 by Roddy Isles
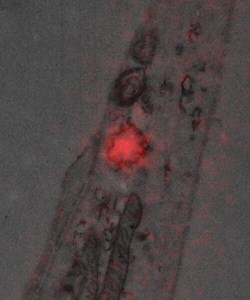
Image shows one of the message-carrying vesicles that may be damaged in SMA. It is visualised in cultured neurone cells by both fluorescence microscopy (in red), to allow analysis of their rapid movement in live cells, and superimposed on an electron microscope image (in grey) of the same vesicle to demonstrate their membrane structure.)
Researchers at the Universities of Dundee and St Andrews have produced new insights into two muscle wasting diseases, including `floppy baby syndrome’ or Spinal Muscular Atrophy,the leading genetic cause of death in children.
Their findings have cast further light on how the genetic defect that causes SMA actually works, an important step in looking for cures.
Spinal Muscular Atrophy (SMA), or ‘floppy baby syndrome’, is the leading genetic cause of death in children. It is a type of motor neuron disease, affecting 1 in 6000 births. The most severely affected children die before the age of two.
“The gene responsible for SMA is known, but it is still not clear how problems with this gene damage the cells of the body and why motor neurons, responsible for sending messages to the muscles, are particularly sensitive,” said Dr Judith Sleeman, of the Biomedical Sciences Research Complex in St Andrews.
In their study, carried out with colleagues at the University of Ottawa and now published in the Journal of Cell Science, the team focussed on the role of the Survival Motor Neurons (SMN) protein, which is made by all cells of the body.
SMN is essential to make parts of a molecular machine called the spliceosome. The spliceosome is needed by the cell to correctly interpret its genetic information. SMN is also needed to help carry genetic messages to different parts of the cell. It is not yet clear which of these roles goes wrong in SMA.
“We have used new techniques to study different stages in the spliceosome ‘manufacturing’ process,” said Dr Sleeman. “Our results show that some of the proteins involved with SMN in the spliceosome are also required for its role in ferrying genetic messages around inside the cell. This suggests that the two roles for SMN are more closely linked than was previously thought.”
In a separate paper published in Biochemical Journal, the Dundee and St Andrews researchers looked at Myotonic Dystrophy Type 1 (DM1), an inherited form of muscular dystrophy that causes progressive muscle weakness and wasting together with a variety of other symptoms, one of the earliest being cataracts in the lens of the eye.
Their research has established that a particular problem in cells previously thought to have played a key role in causing the symptoms of the condition is unlikely to be the sole cause.
The genetic defect that causes the disease occurs in the code for a gene called DMPK1 and affects the messenger molecule (RNA), which is made by the cell when it needs to use the protein made by the DMPK1 gene. The RNA message then accumulates in clumps in the nucleus of the cell.
Dr Prescott, of the College of Life Sciences, University of Dundee, said, “Previous research has suggested that these clumps of RNA then trap a second protein called Muscleblind-like that is needed for the cell to use some of its other genes correctly, causing the symptoms of Myotonic Dystrophy I.
“In fact our results show that, at least in lens cells, the amount of Muscleblind-like protein found in the RNA clumps is extremely small and that it is not trapped within them, but free to come and go. This suggests that the trapping of Muscleblind-like protein is unlikely to cause the symptoms of DM1 and that research into potential treatments for DM1 should focus on other problems that might be caused by the clumps of DMPK1 RNA.”
“For both of these diseases, the genetic defect that causes them has been known for years, but it's not yet clear how the faults damage cells and causes the symptoms,” said Dr Judith Sleeman.
“The more we can understand about how cells are damaged, the more opportunities there will be to explore sensible new avenues looking for cures. These latest results we have reported are small but important parts of the jigsaw in understanding these diseases and ultimately treating them.”
Roddy Isles
Head of Press
University of Dundee
Nethergate, Dundee, DD1 4HN
TEL: 01382 384910
MOBILE: 07800 581902
EMAIL: r.Isles@dundee.ac.uk